Description
Tesamoreli: Tesamoreli is a synthetic growth hormone-releasing hormone (GHRH) analog. By mimicking the naturally occurring GHRH in the human body, it stimulates the anterior pituitary gland to release growth hormone (GH), thereby increasing the level of serum insulin-like growth factor I (IGF-I). In this way, Tesamoreli can regulate the body's growth hormone axis, affect the secretion and release of growth hormone, and thus have an impact on the body's metabolism, growth and development, as well as various physiological functions. Tesamoreli is mainly used to treat lipid metabolism disorders caused by antiretroviral therapy (ART) in HIV-infected individuals, especially the excessive accumulation of abdominal fat. In addition, it also shows certain potential in improving fat distribution, reducing visceral fat, and enhancing metabolic health. During the treatment of GH deficiency with rhGH, clinical efficacy coexists with adverse side effects. These adverse side effects have prompted researchers to search for new treatment strategies, thus promoting the development of GFR analogs. GFR analogs are expected to maintain a certain therapeutic effect while reducing adverse side effects and improving patients' tolerability. As a synthetic GFR, the development of Tesamoreli is mainly aimed at diseases that may be related to a relative deficiency of GH. Among them, HIV-related lipodystrophy is one of its important potential indications. HIV patients often experience symptoms of lipodystrophy, which not only affects the appearance of patients but may also lead to a series of health problems. Therefore, the development of an effective treatment method is crucial for improving the quality of life of HIV patients. Tesamoreli Structure: Source: PubChem Sequence: YADAI F TNSY RKVL GQLS A RK L LQDI M SRQ QGE S NQ E RGA R ARL Molecular Formula: C221H366N72O67S Molecular Weight: 5136 g/mol CAS Number: 218949-48-5 PubChem CID: 16137828 Synonyms: Egrifta ▎Tesamoreli Research What is the research background of Tesamoreli? When recombinant human growth hormone (rhGH) is used in the treatment of growth hormone (GH) deficiency, although it has clinical effectiveness, it is also accompanied by various adverse side effects. This situation has prompted the development of human growth hormone-releasing factor (GFR) analogs, as they may have better tolerability. Tesamoreli is a synthetic GFR that has been developed as a potential treatment for a variety of diseases potentially related to a relative deficiency of GH, including HIV-related lipodystrophy. What is the mechanism of action of Tesamoreli? Mechanism of action on HIV-associated non-alcoholic fatty liver disease (NAFLD) Regulating gene expression: In HIV-associated NAFLD, through gene set enrichment analysis, it was found that Tesamoreli increased the expression of the signature gene set related to oxidative phosphorylation in the liver, while reducing the expression of gene sets related to inflammation, tissue repair, and cell division [1]. Specifically, Tesamoreli downregulated the liver gene sets related to inflammation, tissue repair, and cell division, indicating that it exerts its effect by regulating the expression of these genes. Inhibiting key mediators: Tesamoreli can inhibit key angiogenesis, fibrosis, and pro-inflammatory mediators. For example, it can significantly reduce the levels of vascular endothelial growth factor A (VEGFA), transforming growth factor β1 (TGFB1), and macrophage colony-stimulating factor 1 (CSF1). In participants receiving Tesamoreli treatment, the decrease in plasma VEGFA and CSF1 was associated with a decrease in the non-alcoholic fatty liver disease activity score, and the decrease in TGFB1 and CSF1 was associated with a decrease in the gene-level fibrosis score[2]. As a regulator of monocyte recruitment and activation, CSF1 may become an innovative therapeutic target for HIV-associated NAFLD. Mechanism of action on peripheral nerve injury: In the treatment of peripheral nerve injury, Tesamoreli is believed to enhance axonal regeneration, reduce muscle atrophy, and improve functional outcomes. Current research shows that Tesamoreli may exert its effect by promoting the process of nerve regeneration and repair. The specific mechanism of action is not yet fully understood, but it may involve the regulation of nerve growth factors, the promotion of axonal growth, and myelination, etc. [3]. What is the specific mechanism by which Tesamoreli reduces visceral fat and liver fat in HIV-infected individuals? Regulatory effect on growth hormone Stimulating growth hormone secretion: Tesamoreli is a synthetic growth hormone-releasing hormone (GHRH), which can stimulate the secretion of growth hormone. In HIV-infected individuals, the secretion of growth hormone may be affected, and Tesamoreli can promote the release of growth hormone from the anterior pituitary gland by mimicking the action of GHRH[4-6]. Regulating fat metabolism: Growth hormone plays an important role in fat metabolism. It can promote fat breakdown, increase the oxidation of fatty acids, and reduce fat accumulation. By stimulating the secretion of growth hormone, Tesamoreli may indirectly regulate fat metabolism and reduce the accumulation of visceral fat and liver fat [4-6]. Impact on liver gene expression Changing gene pathways: Studies have found that Tesamoreli can increase the expression of the signature gene set related to oxidative phosphorylation in the liver, while reducing the expression of gene sets related to inflammation, tissue repair, and cell division [1]. Affecting genes related to the prognosis of hepatocellular carcinoma: Tesamoreli can also regulate the gene sets related to the prognosis of hepatocellular carcinoma, upregulating the gene sets related to a good prognosis and downregulating the gene sets related to a poor prognosis, respectively [1]. These changes in gene expression may be related to the effect of Tesamoreli on reducing liver fat. Relationship with fibrosis-related gene scores: In participants treated with Tesamoreli, these changes in liver expression were associated with improved fibrosis-related gene scores. This indicates that Tesamoreli may improve the fibrotic condition of the liver by regulating liver gene expression, thereby reducing liver fat [1]. Impact on plasma proteins Inhibiting key mediators: Tesamoreli can significantly reduce the levels of plasma proteins such as vascular endothelial growth factor A (VEGFA), transforming growth factor beta 1 (TGFB1), and macrophage colony-stimulating factor 1 (CSF1) [1]. These proteins are related to angiogenesis, fibrosis, and inflammation, and the inhibition of them by Tesamoreli may help to reduce liver fat and inflammation. Correlation with disease scores: In participants treated with Tesamoreli, the decrease in plasma VEGFA and CSF1 was associated with a decrease in the non-alcoholic fatty liver disease (NAFLD) activity score, and the decrease in TGFB1 and CSF1 was associated with a decrease in the gene-level fibrosis score[1]. This indicates that the regulation of these plasma proteins by Tesamoreli may be related to the improvement of liver disease conditions. Source:PubMed[1] What are the applications of Tesamoreli? Effects on HIV-infected individuals Reducing visceral fat and liver fat: Some studies have shown that in HIV-infected individuals receiving integrase inhibitor (INSTIs) treatment, the increase in visceral adipose tissue (VAT) is a concern, because VAT is associated with downstream comorbidities such as non-alcoholic fatty liver disease (NAFLD). Tesamoreli, a growth hormone-releasing hormone analog, has been shown to reduce VAT in HIV-infected individuals with lipohypertrophy by more than 15% within 6 months [7]. In a placebo-controlled trial of 61 participants with HIV-associated NAFLD, a post-hoc analysis of individuals receiving INSTIs treatment found that after 12 months, the VAT in the placebo group increased by 10.8%, while the VAT in the Tesamoreli treatment group decreased by 8.3% overall. In addition, the liver fat fraction (HFF) in the Tesamoreli treatment group decreased by 31% relative to the baseline, which was significantly higher than that in the placebo treatment group [7]. Improving fat quality: In HIV-infected individuals, in patients with central obesity who used Tesamoreli, the density of visceral fat (VAT) and subcutaneous fat (SAT) increased, and this increase was independent of changes in fat mass, indicating that Tesamoreli can also improve the quality of VAT and SAT in this group of patients [8]. Impact on immune function: Long-term use of Tesamoreli can reduce the markers of T cell and monocyte/macrophage activity in the circulation of HIV-infected individuals and downregulate the immune pathways in the liver. Specifically, compared with the placebo, Tesamoreli reduced the circulating concentrations of 13 proteins, including four chemokines, two cytokines, four T cell-related molecules, as well as arginase-1, galectin-9, and hepatocyte growth factor. Network analysis showed that there were close interactions between the gene pathways responsible for reducing these proteins, and targeted transcriptomics confirmed the downregulation signal of the immune pathways in the liver [9]. Role in non-alcoholic fatty liver disease Reducing liver fat and preventing the progression of fibrosis: In HIV-associated non-alcoholic fatty liver disease, Tesamoreli has been proven to reduce liver fat and prevent the progression of fibrosis. Researchers conducted a focused evaluation of 9 plasma proteins corresponding to the top leading-edge gene sets in the differentially regulated gene sets and found that Tesamoreli led to a significant reduction in vascular endothelial growth factor A (VEGFA), transforming growth factor β1 (TGFB1), and macrophage colony-stimulating factor 1 (CSF1). In participants treated with Tesamoreli, the decrease in plasma VEGFA and CSF1 was associated with a decrease in the non-alcoholic fatty liver disease activity score, and the decrease in TGFB1 and CSF1 was associated with a decrease in the gene-level fibrosis score. As a regulator of monocyte recruitment and activation, CSF1 may become an innovative therapeutic target for non-alcoholic fatty liver disease in HIV[2]. Impact on the liver transcriptome profile: Using gene set enrichment analysis, it was found that Tesamoreli increased the liver expression of the signature gene set related to oxidative phosphorylation and reduced the liver expression of gene sets related to inflammation, tissue repair, and cell division. In addition, Tesamoreli also upregulated and downregulated the selected gene sets related to good and poor prognosis of hepatocellular carcinoma, respectively. In participants treated with Tesamoreli, these changes in liver expression were associated with improved fibrosis-related gene scores[2]. As a synthetic growth hormone-releasing hormone analog, Tesamoreli has demonstrated therapeutic potential in multiple aspects. In conclusion, a large number of studies have confirmed that it can effectively regulate the secretion of growth hormone and improve the body's metabolic function. In the treatment of HIV infection-related diseases, it has a significant effect on reducing abdominal fat accumulation. By stimulating the secretion of growth hormone and optimizing fat metabolism, it reduces the amount of visceral fat without affecting lean body mass, playing a positive role in adjusting the body composition of patients. In the treatment of non-alcoholic fatty liver disease, some studies have also shown that it can reduce the liver fat content. The significance of Tesamoreli is profound. For HIV-infected patients, it provides an effective means to improve the fat metabolism disorders caused by the disease and treatment, improves the quality of life of patients, helps to relieve the psychological pressure caused by changes in appearance, and enhances their sense of social integration. About The Author The above-mentioned materials are all researched, edited and compiled by Cocer Peptides. Scientific Journal Author Stanley T L is a scholar at Harvard University, engaged in research and teaching at institutions such as Harvard Medical School and Massachusetts General Hospital. His research fields include endocrinology and metabolism, infectious diseases, immunology, microbiology, and nutrition and dietetics. He has conducted in-depth explorations in these fields, providing theoretical bases for the diagnosis, treatment, and prevention of related diseases. At the same time, he closely cooperates with organizations such as Harvard Medical School and Massachusetts General Hospital for Children, combining theory with clinical practice and making contributions to the medical cause. Stanley T L is listed in the reference of citation [4].
▎Relevant Citations [1] Fourman L T, Billingsley J M, Agyapong G, et al. Effects of tesamorelin on hepatic transcriptomic signatures in HIV-associated NAFLD[J]. Jci Insight, 2020,5(16).DOI:10.1172/jci.insight.140134. [2] Fourman L T, Stanley T L, Billingsley J M, et al. Delineating tesamorelin response pathways in HIV-associated NAFLD using a targeted proteomic and transcriptomic approach[J]. Scientific Reports, 2021,11(1).DOI:10.1038/s41598-021-89966-y. [3] Jaimie T S. Tesamoreli Therapy to Enhance Axonal Regeneration, Minimize Muscle Atrophy and Improve Functional Outcomes Following Peripheral Nerve Injury and Repair[J]. 2015. [4] Rahman F, McLaughlin T, Mesquita P, et al. Effect of tesamorelin in people with HIV with and without dorsocervical fat: Post hoc analysis of phase III double-blind placebo-controlled trial[J]. Journal of Clinical and Translational Science, 2022,7(1).DOI:10.1017/cts.2022.515. [5] Mateo M G, Gutierrez M D M, Domingo P. Tesamoreli for the treatment of excess abdominal fat in HIV-1-infected patients with lipodystrophy.[J]. Expert Review of Endocrinology & Metabolism, 2011,6(1):21-30.DOI:10.1586/eem.10.83. [6] Stanley T L, Falutz J, Marsolais C, et al. Reduction in Visceral Adiposity Is Associated With an Improved Metabolic Profile in HIV-Infected Patients Receiving Tesamoreli[J]. Clinical Infectious Diseases, 2012,54(11):1642-1651.DOI:10.1093/cid/cis251. [7] McLaughlin T, Grinspoon S K, Stanley T, et al. 1499. Tesamoreli Reduces Visceral Adipose Tissue and Liver Fat in INSTI-Treated Persons with HIV[J]. Open Forum Infectious Diseases, 2023,10(2):500-1334.DOI:10.1093/ofid/ofad500.1334. [8] Lake J E, La K, Erlandson K M, et al. Tesamoreli improves fat quality independent of changes in fat quantity[J]. Aids, 2021,35(9):1395-1402.DOI:10.1097/QAD.0000000000002897. [9] Stanley T L, Fourman L T, Wong L P, et al. Growth Hormone Releasing Hormone Reduces Circulating Markers of Immune Activation in Parallel with Effects on Hepatic Immune Pathways in Individuals with HIV-infection and Nonalcoholic Fatty Liver Disease[J]. Clinical Infectious Diseases, 2021,73(4):621-630.DOI:10.1093/cid/ciab019. ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE SOLELY FOR INFORMATION DISSEMINATION AND EDUCATIONAL PURPOSES. The products provided on this website are intended exclusively for in vitro research. In vitro research (Latin: *in glass*, meaning in glassware) is conducted outside the human body. These products are not pharmaceuticals, have not been approved by the U.S. Food and Drug Administration (FDA), and must not be used to prevent, treat, or cure any medical condition, disease, or ailment. It is strictly prohibited by law to introduce these products into the human or animal body in any form.

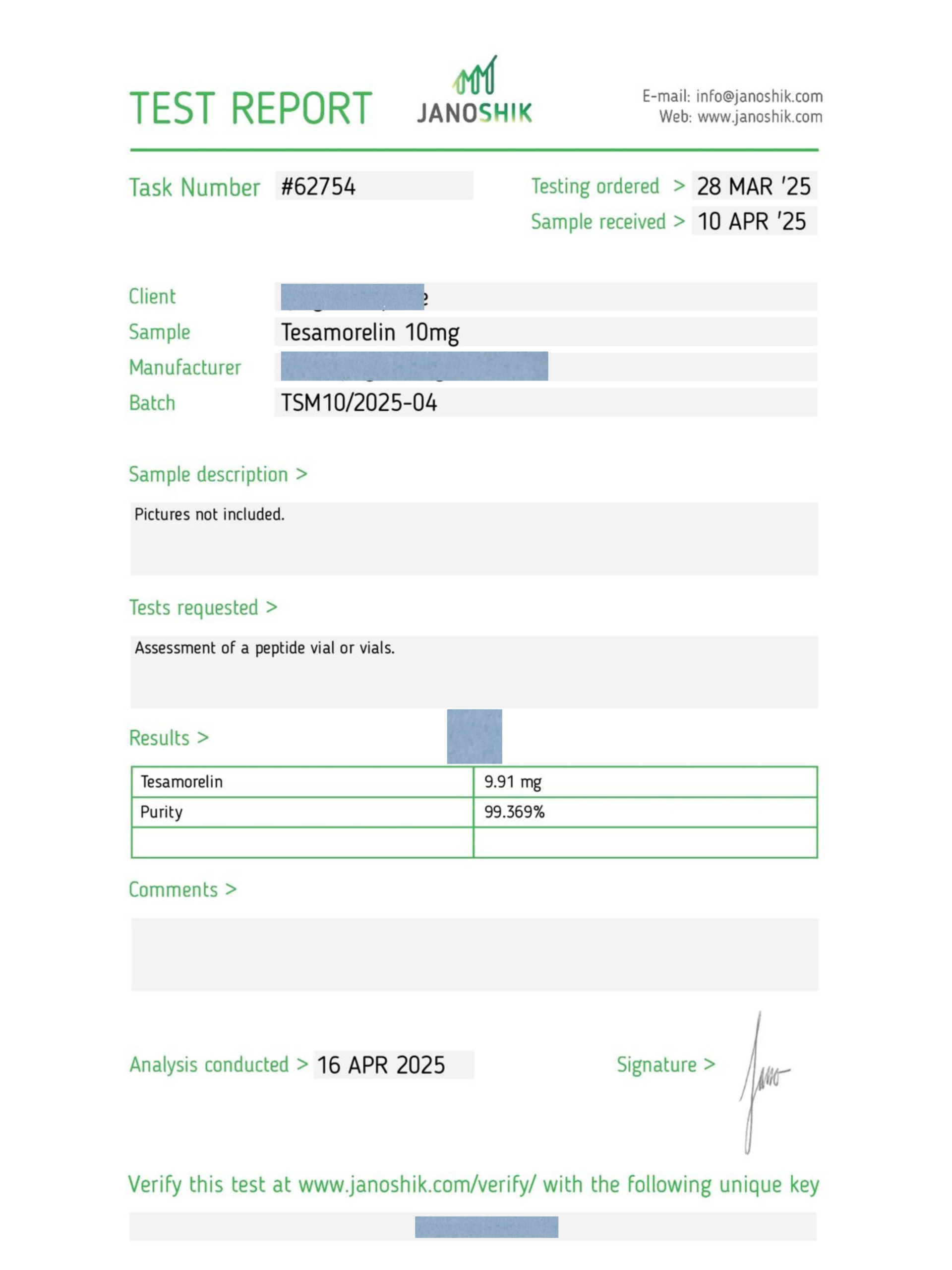

Reviews
There are no reviews yet.